US8287890B2 HYDROPHILIC COATING
US8287890B2
Patent No.: US 8,287,890 B2
Date of Patent: Oct. 16, 2012
HYDROPHILIC COATING
Inventor: Richard K. Elton,
Assignee: C.R. Bard, Inc., Murray Hill, NJ (US)
A hydrophilic, lubricious coating for a substrate includes a first coating layer having a cross-linked polyurethane or polyurea complexed with poly(ethylene oxide) formed by reacting a mixture of an isocyanate, a polyol or polyamine, and a poly(ethylene oxide), and a second coating layer having a cross-linked polyurethane or polyurea complexed with polyvinylpyrrolidone formed by reacting a mixture of an isocyanate, a polyol or polyamine, and a polyvinylpyrrolidone. The first layer is substantially covered by the second layer and the second layer at least partially interpenetrates the first layer.
基材用の親水性、潤滑性コーティングは、イソシアネート、ポリオールまたはポリアミン、およびポリ(エチレンオキシド)の混合物を反応させることによって形成される、ポリ(エチレンオキシド)と複合体を形成した架橋ポリウレタンまたはポリ尿素を有する第1のコーティング層を含み、 イソシアネート、ポリオールまたはポリアミン、およびポリビニルピロリドンの混合物を反応させることによって形成される、ポリビニルピロリドンと複合体を形成した架橋ポリウレタンまたはポリ尿素を有する第2のコーティング層。 第1の層は第2の層によって実質的に覆われ、第2の層は少なくとも部分的に第1の層に相互浸透する。
【Ex1】
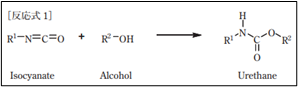
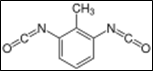
3.60g; toluene diisocyanate adduct of trimethylolpropane / Desmodur L 67 MPA/X;
Desmodur® L 67 MPA/X | Covestro
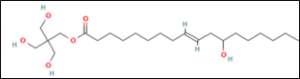
1.34g; modified castor oil polyol / Polycin 12;
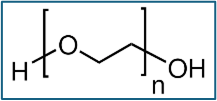
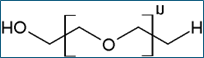
150g; 2.5% (w/w) solution of ˜300,000 molecular weight poly(ethylene oxide) in methylene bromide
/ Sigma Aldrich solvent
595g; methylene bromide.
1% (w/w) concentration,NCO/OH ratio of 1.3,polyurethane solids to poly(ethylene oxide) of 1.0.
1.10g ; toluene diisocyanate adduct of trimethylolpropane / Desmodur L 67 MPA/X;
2.25g: polyester polyols / Desmophen 1800;
Desmophen® 1800 | Covestro
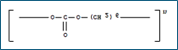
100g: polyvinylpyrrolidone in methylene bromide /Kollidon 90F
(dried for 90 minutes at 250°F. prior to placing in solution),
Kollidon® 90 F | Povidones, Copovidones, Crospovidones | BASF Pharma
377 g of methylene bromide.
1.65% (w/w) concentration, NCO/OH ratio of 1.3, polyurethane solids to polyvinylpyrrolidone of 0.4.
A catheter containing a inflated PET balloon;2 inches/second, 0.3 inches/second.
dry in 20 minutes, 165°F. for a period of 4 hours.
The catheter was then coated with in the above mentioned polyurethane/PVP top coating,
using the same dipping and baking conditions.
【Ex2】
25.6g: toluene diisocyanate adduct of trimethylolpropane / Desmodur L 67 MPA/X;
42.4g: polyester polyol / Desmophen 1800;
1200g: 5% (w/w) poly(ethylene oxide) / Polyox WSR N80 NF
![]()
3528g: methylene bromide.
2.5% (w/w) , NCO/OH 1.3 , polyurethane solids to poly(ethylene oxide) of 1.0.
1.12g: toluene diisocyanate adduct of trimethylolpropane/ Desmodur L 67 MPA/X;
0.21g: castor oil polyol, / Polycin 12;
322g: methylene bromide
5.0g: polyvinylpyrrolidone/ Kollidon 90F.
1.7% (w/w) concentration, NCO/OH ratio of 2.6, polyurethane solids to polyvinylpyrrolidone of 0.19.
A catheter containing an inflated PET balloon
1 inches/second, 0.5 inches/second. 20 minutes, 165°F. for a period of 1 hour.
polyurethane/PVP top coating, using the same dipping conditions, 165° F. for a period of 4 hours.
【Ex3】
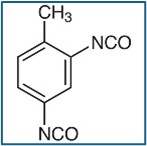
17.0g: toluene diisocyanate adduct of trimethylolpropane/ Desmodur L 67 MPA/X;
1.11g: polyetheramine /Jeffamine EDR148;
Jeffamine EDR 148 | CASE | Azelis
![]()
200g: 5% (w/w) poly(ethylene oxide) in methylene bromide / Alkox R400
アルコックス® Eグレード,Rグレード | 明成化学工業株式会社 (meisei-chem.co.jp)
507g: 1,3-dioxolane.
3.1% (w/w) NCO/NH ratio of 1.1, polyurea solids to poly(ethylene oxide) of 1.25.
5.99g: toluene diisocyanate adduct of trimethyloipropane/ Desmodur L 67 MPA/X;
2.99g: castor oil polyol / D B Oil;
Specialty Chemical and Fine Ingredient Distributor – Palmer Holland
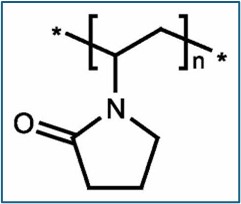
200g: 5% polyvinylpyrrolidone in 1,3-dioxolane / Plasdone 90
※dried for 90 minutes at 250°F
plasdone™ k-90 povidone (ashland.com)
196g: 1,3-dioxolane.
4.2% (w/w) NCO/OH 1.3, polyurethane solids to polyvinylpyrrolidone of 0.7.
A catheter containing an inflated PET balloon
1.5 inches/second, 0.5 inches/second.
dry 30 minutes, 165°F. 4 hours.
the same dipping and baking conditions.
【Ex4】
11.7g: castor oil modified polyisocyanate / Vorite 63;
https://www.palmerholland.com/All-Products/VORITE-63
&bsp;
8.26g: polyether polyol / Poly THF 650;
PolyTHF® – Polytetrahydrofuran (basf.com)
![]()
400g: 2.5% (w/w) poly(ethylene oxide) in methylene bromide / Alkox R400
2080g: methylene bromide
1.2% (w/w), NCO/OH ratio of 1.3, polyurethane to poly(ethylene oxide) of 2.0.
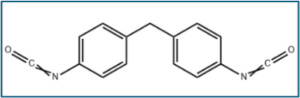
3.1g: polyisocyanate/ Baytec MP-080;
Baytec® MP-080 | Covestro
0.9g: polyetheramine / Jeffamine ED 600;
JEFFAMINE® ED-600 Polyetheramine (ku.edu.tr)
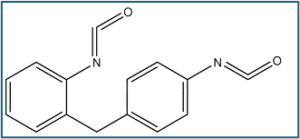
200g: 5% polyvinylpyrrolidone in acetonitrile /Plasdone 90
※dried for 120 minutes at 225°F.
85.7g: acetonitrile.
4.9% (w/w) NCO/NH ratio of 1.4, polyurethane solids to polyvinylpyrrolidone of 0.4.
A catheter containing an inflated PET balloon
1.2 inches/second,
0.4 inches/second.
dry 20 minutes, 170°F 4 hours.
【Ex5】
8.7g: aromatic polyisocyanate product Desmodur IL;
Desmodur® ultra IL BA | Covestro
![]()
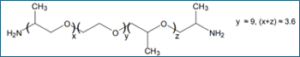
6.4g: polyether polyol Poly THF 1000;
200g: 5% (w/w) poly(ethylene oxide) in 1,3-dioxolane Alkox R400
896g: methylene bromide solvent.
2.25% (w/w) NCO/OH 1.3, polyurethane solids to poly(ethylene oxide) of 1.5.
3.1g: polyisocyanate/ Baytec MP-210;
Baytec Polymers For Elastomers (adhesivesandsealants.com)
MDI終端のポリエーテルプレポリマーで、ポリプロピレングリコールをベース
5.31g: modified castor oil polyol/ Polycin 12;
400g: 5% polyvinylpyrrolidone in acetonitrile /Plasdone 90
(dried for 4 hours at 205° F.),
499g: 1,3-dioxolane.
4.5% (w/w) NCO/OH 1.1, polyurethane solids to polyvinylpyrrolidone o 0.6.
1.0 inches/second, f
0.3 inches/second.
dry 20 minutes, at 175°F. for a period of 3.5 hours.
【Ex6】
26g: diisocyanate adduct of trimethylolpropane, / Desmodur L 67 MPA/X;
4.6g: trifunctional polyetheramine / Jeffamine T-403;
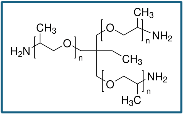
400g: 5% (w/w) poly(ethylene oxide) in methylene bromide / Alkox 8150
1329g: acetonitrile
2.5% (w/w) NCO/NH 1.3, poly(ethylene oxide) of 1.2.
10.0g: diisocyanate adduct of trimethylolpropane, / Desmodur L 67 MPA/X′
3.3g: polyetheramine / Jeffamine ED 600;
JEFFAMINE® ED-600 Polyetheramine (ku.edu.tr)
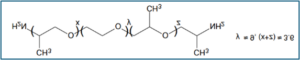
400g: 5% polyvinylpyrrolidone in methylene bromide /Kollidon 90F,
(dried overnight at 180° F),
1405g: methylene bromide.
1.65% (w/w) NCO/NH 1.3, polyurea to polyvinylpyrrolidone of 0.5.
2.0 inches/second,
0.5 inches/second.
dry in 20 minutes, 150° F. 8 hours.
COMPARATIVE EXAMPLES
【Com1】
A balloon catheter as described in Example 1 was coated in the polyurethane/PVP top coating as described in Example 1, but without first coating with the polyurethane/PEO primer coating.
noticeably less lubricious.
【Com2】
13.8g: toluene diisocyanate adduct of trimethylolpropane,/Desmodur L 67 MPA/X;
6.1g: modified castor oil polyol, / Polycin 12
364g: butyl acetate
4% (w/w) NCO/NH 1.1
A polyurethane/PVP top coating was prepared substantially as described in Example 1
1.10g: toluene diisocyanate adduct of trimethylolpropane,/ Desmodur L 67 MPA/X;
2.25g: polyester polyol, / Desmophen 1800;
Desmophen® 1800 | Covestro
100g: 5% polyvinylpyrrolidone in methylene bromide /Kollidon 90F
(dried for 90 minutes at 250° F. before placing in solution), available as,; and
377g: methylene bromide.
1.65% (w/w) NCO/OH ratio of 1.3, polyurethane solids to polyvinylpyrrolidone of 0.4.
2 inches/second,
0.3 inches/second.dry 20 minutes, 165° F. 4 hours.
a noticeable loss of lubricity was observed, compared to a balloon catheter coated using a polyurethane/PEO primer as described in Example 1.
This demonstrated that even with a polyurethane primer which adhered to the balloon, the lubricious topcoat was less durable compared to a primer coat comprising polyurethane/PEO or polyurea/PEO composition.
The present coating can be dried and remoistened repeatedly while retaining its lubricating properties.

