Manufacturing Methods of Silicone Monomers 1
Manufacturing Methods of Silicone Monomers
By Isao Nakajima (1976, Vol. 34, No. 8, pp. 583–588)
2. Manufacturing Methods of Silicone Monomers
Organohalosilanes are the most practically important monomers for the production of silicone materials. When organohalosilanes with different functionalities react with water, either alone or in combination, they are converted into polyorganosiloxanes—intermediate compounds used in silicone production.

From these intermediates, the final silicone products are derived through polymerization, condensation, and various secondary processing methods such as emulsification, addition of fillers, crosslinking agents, and other additives.
Thus, the economic, efficient, and safe production of organohalosilanes as starting materials is the first key to the success of the silicone industry. Over the past decades, companies such as Dow Corning and GE have devoted significant efforts to the development and rationalization of monomer production technologies.
The economic production of organohalosilanes with various functional groups is not straightforward. Furthermore, controlling the reaction to yield the desired ratios of differently functionalized organohalosilanes as required by the end product is extremely difficult. The most important compounds industrially are functional siloxanes, and achieving high yields of these is of critical importance.
This section classifies the currently practiced manufacturing methods according to convention and reviews the features and significance of each.
The main production methods for RₙSiX₄₋ₙ type organosilanes are:
1. Using organometallic compounds
2. Direct synthesis – direct reaction between silicon and organic halides
3. Reactions with hydrocarbons and hydrosilanes (HSi=)
3-1. Addition reactions with unsaturated hydrocarbons
3-2. Substitution reactions with hydrocarbons or halogenated hydrocarbons
4. Exchange reactions
Depending on the type and amount of required monomer, a suitable synthesis method is selected from among these.
2.1. Methods Using Organometallic Compounds
This is the oldest method in organosilicon compound synthesis, in which negatively charged groups such as chlorine or alkoxy attached to a silicon atom are replaced by organic groups from organometallic compounds.
2.1.1. Grignard Synthesis
Grignard reactions have been used in the synthesis of organosilicon compounds since 1904, when Kipping first applied the method. For nearly 70 years, it was the most effective technique to form Si–C bonds. Before the emergence of the direct method, Dow Corning industrialized silicone production using this technique. Though largely replaced by the direct method today, Grignard synthesis remains fundamental in laboratory synthesis and is still used industrially for producing special silanes and carbon-functional silanes.
![]()
This reaction can proceed in one or two steps, allowing various silanes to be synthesized freely and in good yield. However, it involves the use of large quantities of ethers and generates significant by-products like magnesium chloride, which raises economic, safety, and environmental concerns—making it unsuitable for large-scale production.
2.1.2. Methods Using Organolithium Compounds
Although relatively recent, methods using organolithium compounds are also employed for introducing organic groups into silicon atoms, similar to Grignard reagents.
![]()
An older method, the Wurtz reaction, allows the synthesis of organohalosilanes by reacting organic halides and halosilanes using alkali metals.
![]()
Compared to the Grignard and direct methods, these methods are less practical. While they have higher reactivity than Grignard reagents, they are more difficult to control—especially for producing partially substituted compounds—and typically offer lower yields. However, they are less affected by steric hindrance, enabling the synthesis of compounds like tetra-isobutylsilane and tetra-naphthylsilane, which are difficult to obtain via Grignard reagents and SiCl₄.
2.1.3. Other Methods
Recently, organoaluminum compounds have also been used for introducing organic groups to silicon atoms.
![]()
Although these reactions are less reactive than Grignard reagents and generally require higher temperatures, they are suitable for adding more alkyl groups to silanes that already contain alkyl groups. However, they react poorly with SiCl₄.
2.2. Direct Method
In 1941, E. G. Rochow discovered a method to produce organochlorosilanes by reacting organic halides with silicon, thereby establishing the industrial production method for silicone monomers. The vast development of the silicone industry owes much to this method.
The so-called “direct method” involves reacting powdered metallic silicon and a metal catalyst (mainly copper) with halogenated hydrocarbons at 250–500°C.
The primary reaction is:
![]()
In practice, several side reactions also occur. While both fixed-bed and fluidized-bed reactors can be used, the industrial standard is the fluidized-bed reactor, with some forced-stirring reactors also adopted.
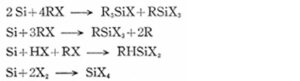
Methyl- and phenyl-based chlorosilanes—key raw materials for silicone—are all produced using this direct synthesis method.
To be continued

